Step into the assembly workshop at Kohope Medical Needle Industrial Park, and you’ll notice a stark departure from traditional manufacturing facilities. Instead of bustling crowds, fully automated assembly equipment operates continuously, completing needle attachment and locking mechanisms for safety syringes in remarkably short cycles. These products are destined for over 50 countries and regions worldwide.
Founded in 2005, Kohope stands as one of China’s pioneering medical supplies company specializing in the research and manufacturing of safety needlestick prevention products. Over the past two decades, the company has maintained focused investment in safety injection, blood collection, infusion, drug preparation, and pharmaceutical packaging materials, developing a comprehensive product portfolio. Today, the enterprise holds numerous core technology patents and operates a dedicated R&D team, earning recognition as a National High-Tech Enterprise.

Scaling Production Capacity
As market demand accelerated, production capacity became critical to the company’s continued growth trajectory. This year, Kohope’s newly constructed medical industrial park commenced operations, expanding overall capacity to approximately five times previous levels. According to Mrs. Wang, General Manager, the company’s daily production capacity for safety needlestick prevention products now exceeds one million units, meeting the demands of large-scale clients.

Smart Manufacturing Transformation
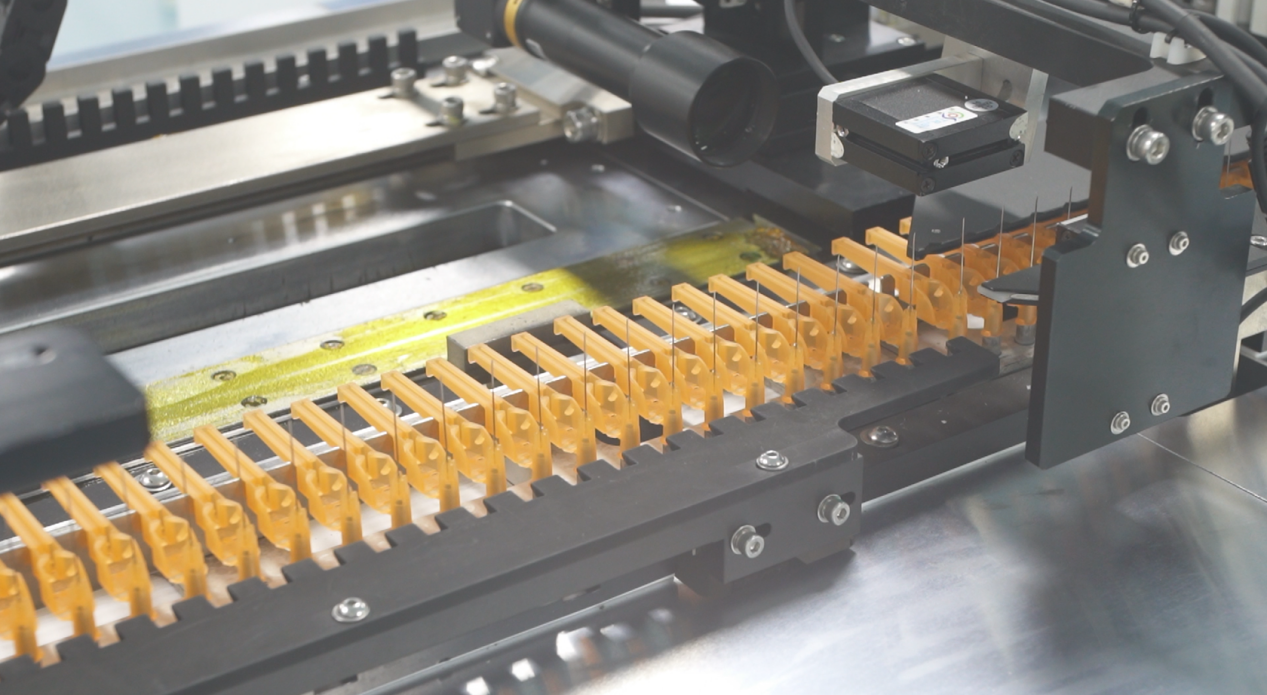
Behind this capacity expansion lies a systematic production model upgrade. In recent years, Kohope has pursued comprehensive intelligent manufacturing transformation, progressively achieving full-process automation from component manufacturing through final assembly. Through implementation of AI-powered sensing and control systems, data visualization management platforms, and big data analytics tools, the company has achieved over 90% networking rate for critical equipment such as injection molding machines. Deep integration between MES (Manufacturing Execution System) and ERP systems enables automatic production scheduling and remote monitoring, significantly reducing manual intervention.
Quality Control Innovation
For quality assurance, the enterprise has deployed intelligent inspection equipment in injection molding operations, conducting online testing and real-time sorting of critical parameters including syringe sealing integrity and needle angles, replacing traditional manual sampling methods. Performance data indicates that following intelligent transformation completion, product defect rates decreased approximately 40%, while overall production efficiency improved roughly 200%.
Currently, Kohope is advancing construction of a “Cloud Intelligence Computing” system spanning R&D, production, and supply chain operations, further enhancing equipment coordination and management precision to support scalable, stable delivery.
Market Resilience and Product Innovation
Amid heightened external uncertainty, Kohope has addressed challenges through new market expansion and accelerated product upgrades. From January through August of this year, company sales increased 23% year-over-year, with certain client orders already scheduled through May 2026, demonstrating strong market resilience.
Regarding product structure, while maintaining stability in safety syringes and other foundational products, the company continues advancing high-value-added offerings. The disposable safety arterial blood collection needles manufacturer has optimized structure and sealing processes to address clinical blood collection safety requirements, enhancing usage safety and sampling accuracy. Products under development—including pre-filled retractable weight management injection systems and ultra-clean drug preparation devices—target consumer healthcare and high-frequency clinical application scenarios.

Strategic Vision
From a single syringe to global market presence, Kohope’s development trajectory reflects Chinese medical device manufacturers’ sustained exploration in automation, digitalization, and product advancement. Looking ahead, the company plans to extend upstream into raw materials and downstream into medical service support while consolidating its core competitive advantages as a syringe manufacturer. The objective: constructing an integrated industrial system spanning production, supply, and sales, continuously elevating its professional reputation and comprehensive competitiveness within the global healthcare sector.





