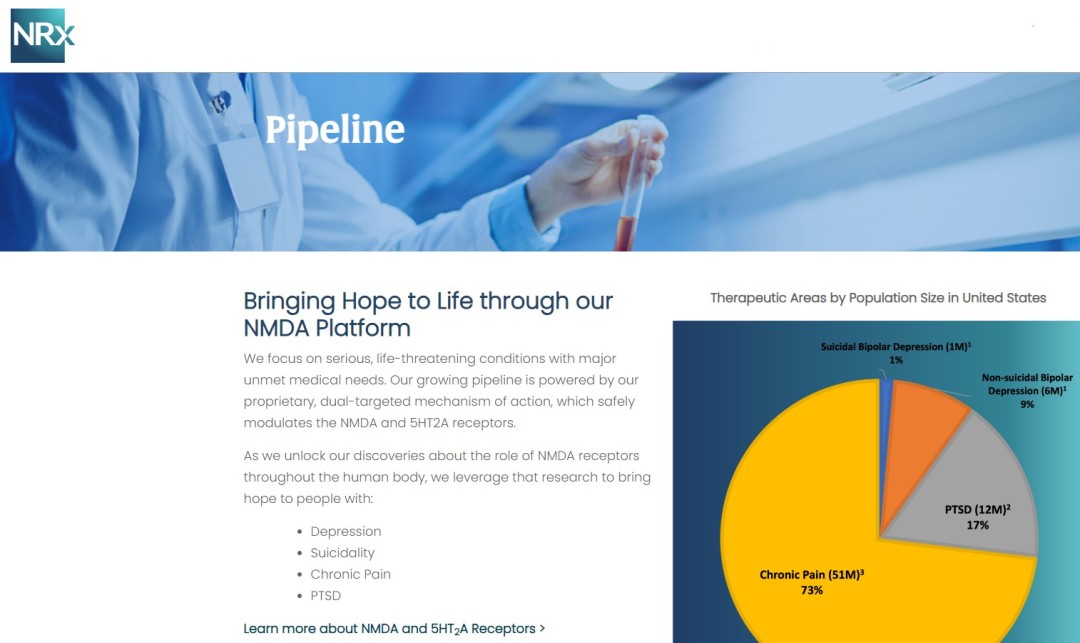
- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
- Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
- D. Boral Analyst Report on NRXP $34 Price Target.
- Type C Meeting with the FDA Demonstrates a Path to New Drug Application with Real World Data and Broader Proposed Indication for NRX-100 (ketamine).
- In-Person Meeting attended with FDA Division of Psychiatry Products and Leadership of the FDA Center for Drug Evaluation and Research (CDER).
- On Path to Application for New Drug Approval of NRX-100 Under Fast Track Designation Based on Existing Clinical Trial Data and Real World Evidence.
- NRXP Will Seek Broader Indication to Serve Patients with Treatment-Resistant Depression Who May Have Suicidality Rather Than the Subset with Suicidality.
- 70,000 Patient Data on Real World Use of Ketamine for Treatment of Suicidal Depression to be Submitted to the FDA in Support of NRX-100 Approval
- Analyses Suggested Clinical Response is Consistent with Prior Randomized Trial Data and Compares Favorably to Currently-Approved Products.
- NRXP and neurocare Group AG Announce Joint Offering of Neuroplastic Therapy Targeting Depression, PTSD and Other Mental Health Afflictions.
- Elimination of All Balance Sheet Debt Following Equity Conversion.
- New Pipeline Indication for Augmentation of Transcranial Magnetic Stimulation.
- Applied for Use of KETAFREE™ as a Proprietary Product Name as First Preservative-Free Ketamine Formulation.
- Current Worldwide Generic Ketamine Market Estimated at $750 Million Per Year.
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
D. Boral has issued an Analyst Report on NRXP with a Buy and $34 Price Target. The full report may be accessed at this direct link: https://www.nrxpharma.com/wp-content/uploads/2025/11/HOPE-Therapeutics-NRXP-Executes-Florida-Roll-out-of-Ampa-O.pdf
Path to New Drug Application with Real World Data and Broader Proposed Indication for NRX-100 (ketamine) Following Type C FDA Meeting
On February 17th NRXP announced that it has completed an in-person Type C guidance meeting, together with Osmind, Inc. at the headquarters of the US Food and Drug Administration. The meeting was attended by leaders of the FDA Division of Psychiatry Products, the FDA Office of Neuroscience, and the FDA Center for Drug Evaluation and Research.
Based on oral guidance received at the meeting, NRXP believes it has a path to filing an application for New Drug Approval of NRX-100 (preservative-free ketamine) based on Substantial Evidence of Effectiveness derived from existing data from adequate and well controlled trials together with confirmatory evidence from more than 65,000 patients identified in the Real World Evidence dataset. NRXP will additionally seek a broader indication to serve patients with treatment resistant depression in the context of suicidality, rather than only the subset of patients with suicidality.
The Companies will work collaboratively with the FDA in the coming weeks to finalize the statistical analysis protocol for the full 65,000 person Real World Evidence dataset under FDA’s newly published guidance.
In preliminary comments ahead of meeting, FDA advised NRXP that no additional nonclinical data would be required for review of the NRXP New Drug Application and that no bridging studies would be needed to support the NRXP preservative-free formulation compared to the currently-approved preservative-containing formulation of ketamine.
70,000 Patient Data on Real World Use of Ketamine for Treatment of Suicidal Depression to be Submitted to the FDA in Support of NRX-100 Approval
On January 14th NRXP announced that it has licensed Real World Evidence (RWE) drawn from over 70,000 patients in the United States who were treated with either intravenous ketamine or nasal S-ketamine for depression and suicidal ideation. The information is being submitted in support of the NRXP application for Accelerated Approval of NRX-100 (preservative-free ketamine) under Fast Track Designation for Treatment of Suicidal Ideation in Depression and Bipolar Depression. Currently, there is no medicine approved to treat suicidal ideation and the only FDA-approved treatment today is Electroshock Therapy.
A preliminary analysis of a 20,000 patient subset documented rapid resolution of depression and suicidality with initiation of intravenous ketamine. NRXP analyses suggested that clinical response to intravenous ketamine is consistent with prior randomized trial data and compares favorably to currently-approved products. Results from an upcoming analysis of the full 70,000 patient Real World Data set will be presented to the FDA in support of Accelerated Approval.
NRXP and neurocare Group AG Announce Joint Offering of Neuroplastic Therapy Targeting Depression, PTSD and Other Mental Health Afflictions
On January 5th NRXP announced a partnership with neurocare Group AG to create a nationwide network of clinics to offer integrated neuroplastic care for the treatment of Depression, PTSD, and other serious mental health disorders. Pilot programs have shown that combining Transcranial Magnetic Stimulation (TMS) with ketamine and other neuroplastic drugs, along with hyperbaric oxygen therapy and supportive psychotherapy has resulted in a high rate of remission among First Responders with PTSD and Depression.
Recent scientific publications have demonstrated that integration of TMS with neuroplastic drugs has achieved unprecedented response of 87% and remission of 72% in patients with treatment-resistant depression. NRXP and neurocare are additionally exploring joint regulatory paths including clinical trials to address the treatment of Bipolar Depression, Autism, and Traumatic Brain Injury with TMS and neuroplastic drugs, such as NRX-101.
The NRXP program will integrate existing neurocare clinics with HOPE Therapeutics clinics and will engage the already-installed base of 400+ Apollo® Transcranial Magnetic Stimulation (TMS) machines nationwide.
Elimination of All Balance Sheet Debt Following Equity Conversion
On December 18th NRXP announced that its remaining $5.4 million debt was repaid through strategic equity conversion in common stock with no additional warrants or adjustment provisions. The debt-free balance sheet sets the stage for accelerated NRXP growth in 2026 with potential drug approvals and clinic expansions.
New NRX-101 Pipeline Indication for Augmentation of Transcranial Magnetic Stimulation
On December 3rd NRXP announced that it has amended its Investigational New Drug filing for NRX-101 (D-cycloserine/lurasidone) to include its use in association with Transcranial Magnetic Stimulation (TMS) for the treatment of depression, including suicidal depression.
In the third quarter, NRXP identified a promising new indication for NRX-101 that potentially offers rapid path to commercialization for this Breakthrough Therapy-designated drug. Recent evidence suggests that NRX-101 may confer a significant added advantage to the clinical results of Transcranial Magnetic Stimulation.
On November 4th Real World Data were presented in conjunction with use of a modern Theta Burst FDA-cleared TMS device and a one day TMS protocol, combined with a single administration of oral DCS. The authors reported 87% clinical response and 72% remission manifesting at 6 weeks after a single day of treatment on the Hamilton Depression Rating Scale with similar findings on other standard test measures.
In addition to containing D-cycloserine, NRX-101 contains a low dose of lurasidone, a medicine already approved for treatment of depression and known to have anti-hallucinatory properties. D-cycloserine by itself is well known to have a low, but measurable potential for inducing low-grade hallucinations, resulting in a labeled contraindication against the use of Seromycin® (D-cycloserine) in patients with depression. This contraindication originally led the founders of NRXP to develop and patent the NRX-101 drug combination, which has now obtained composition of matter patent protection in all major jurisdictions.
Given current trends, NRXP expects that more than 1 million Americans per year may be treated with TMS by the year 2030, creating a substantial new potential market for NRX-101 not previously anticipated. As required by law for Breakthrough Therapy drugs such as NRX-101, NRXP has published an Expanded Access policy for this medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Media Contact
Company Name: NRx Pharmaceuticals, Inc.
Contact Person: Matthew Duffy, Chief Business Officer
Email: Send Email
Phone: 484 254 6134
Address:1201 Orange Street Suite 600
City: Miami
State: Florida
Country: United States
Website: https://www.nrxpharma.com/






